by Beate Treffler, market development manager healthcare EMEA, Avient Corporation
In the past two decades, laser plastic welding has taken the manufacturing world by storm. Seen as little more than science fiction at the turn of the century, it now is one of the top technologies to deep-weld polymer materials together in many industries.
Used in the development of complex shaped plastic parts for everything from the automotive market to electronic circuits, it also has become the technology of choice for joining plastics for today’s increasingly advanced medical devices and high-quality seams, as well as adhesive-free assembly. It is being used across multiple application areas, including complex cardiac and drug delivery devices, diagnostic products, wearables, stent assemblies, sterile kits and dialysis systems.
Also known as transmission welding, laser welding is the process of using focused laser radiation to bond two plastic parts, one that is transparent to the laser energy and one that absorbs the energy to create the weld.
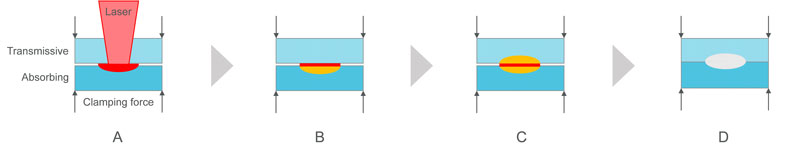
In this process, the two plastics are clamped together, while a focused laser beam passes through the upper laser transmissive part to the interface of the two plastic parts to be assembled (Figure 1; A). The laser light turns into heat energy, which is absorbed by the lower plastic part (B). The heat then moves to the upper plastic part (C). The joint melting pool of both parts creates, under external clamping force, a molten weld seam that results in the two plastic parts being fused together (D). The process causes minimal mechanical stress, creates no surface damage and yields strong, durable and consistent assembly.
New technologies create new challenges
Being such a precise and clean process, it has many advantages over traditional welding techniques. As laser plastic welding does not use mechanical forces to heat and melt parts, it produces hygienically clean surfaces with no contamination, meaning there are no post-production cleaning requirements. Seams are strong and consistent even in plastic components that are just a few millimeters in size. There is minimal mechanical stress to a part, no surface damage and fast cycle times.
With the development of increasingly sophisticated medical devices that are smaller in size, yet provide more sophisticated functionality, the advantages of laser plastic welding help to ensure perfection in form and function to meet these challenging requirements.
However, different polymers interact differently with laser energy, making them more or less suitable for laser welding. As medical devices frequently are made of transparent or translucent materials, special polymer solutions often are used to enhance a polymer’s ability to absorb the laser energy and achieve a good weld for superior and advanced medical devices.
These polymer solutions usually are provided in the form of concentrate – also known as masterbatch – pellets, with the laser welding material optimally dispersed in the polymer matrix. The injection molder then uses the concentrate at a certain dosage, recommended by the provider, with virgin polymer to achieve optimal laser absorption or laser transmission.
The right formulation is key
Laser welding concentrates can be developed for the different polymers traditionally used in the manufacturing of medical devices from polyolefins, TPE, ABS and SAN to engineered resins like PA, PC and PMMA. The color of the two parts to be assembled can be different or similar. The parts can look more opaque or more transparent. For the laser welding concentrate provider, developing the best polymer solution is eventually a formulation exercise that takes into consideration every aspect of the laser welding project: color, polymer, opacity, part thickness, laser wavelength and the required processing parameters.
Another important factor in achieving a good weld is the even distribution of the laser welding material throughout the polymer matrix of the final part. In some cases, a concentrate can be dosed at the injection-molding machine, which then mixes it sufficiently into the polymer melt before molding. However, injection molding machines are not always ideal for dispersing the concentrate into the host polymer.
In some applications, the machine, the material or the part design may cause inconsistent distribution and lead to unreliable welding. In this case, the supplier of polymer solutions can provide ready-to-use polymer/additive formulations in which the laser welding material, along with other pigments or additives, are optimally distributed. The injection molder then can use this all-in-one material without further dilution.
Keeping up with regulatory requirements
Finally, the regulatory aspects of medical device design and manufacture need to be considered and this is much simpler for producers when the raw materials used already are pre-tested against pharmaceutical standards and key certifications are applied. These measures support the compliance of the final device tremendously.
In one example, raw material suppliers that are certified to ISO 13485-2016 for medical devices show that the organization has a Quality Management System (QMS) that is appropriate and effective, meeting all of the EU MDR QMS requirements. It includes documentation like requirements for medical device files, work environment requirements, contamination control requirements, production requirements for cleanliness of products and elaborated risk analysis requirements.
In another example, USP Class testing is one of the most common methods of testing to determine biocompatibility of materials. There are six classes, VI being the most rigorous and aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials (USP 23 pt 87, 88). ISO 10993 also entails a series of standards for evaluating the biocompatibility of medical devices to manage biological risk.
Device manufacturers should look for masterbatches and formulations from companies that have pre-tested raw material ingredients and apply key standards to ensure compliance.
All testing of final devices and raw materials is only as good as the control the device manufacturer puts in place, covering not only the production of the device but also the raw materials used.
Regulations can be continuously met only by using controlled materials. In this respect, change control comprises the whole QMS of a supplier and is equally as important as any testing because it maximizes the relevance of the end-product testing being done for submission, provides confidence during product life cycle and cannot be imposed – that is, products need to be designed with change control in mind.
Growing momentum
Product developers and companies operating in the current laser plastic welding market increasingly are focusing on opportunities in the healthcare sector, always looking to introduce new product designs in the current market landscape, enhancing the overall efficacy and performance of their assembly/joining processes. Indeed, the global laser plastic welding market is forecast to reach a market value of around US $1.5 billion by 2025 with further advancements in the technology continuing to propel demand.
With its multiple benefits, it is clear that laser plastic welding has emerged as an ideal solution to the design and manufacture of complex parts for a host of industrial sectors, but specifically in medical devices, and it seems set to go from strength to strength.
Beate Treffler is the market development manager healthcare EMEA at Avient Corporation, where she manages the development of polymer solutions for pharma packaging, medical and drug delivery devices. These polymer solutions comprise pre-tested and change-controlled masterbatches, as well as ready-to-use formulations. With a degree in chemical engineering, Treffler has more than 25 years’ experience in additives for plastic applications and plastic processing. For more information, visit www.avient.com.


