Long before the first ink drop is jetted onto a plastic product, designers make key decisions that predetermine whether ink printing will be easy or difficult. Adhesion is essentially a superficial phenomenon, depending as it does upon interactions between the liquid ink and the surface of the substrate. Principal factors affecting adhesion are polymer selection (including colorants, fillers and additives), molding process, surface conditions, and storage and handling. Inkjet printing is highly complex, involving many interdisciplinary fields. First and foremost is ink-polymer substrate compatibility. This article will examine the selection of polymers and primary processing to achieve robust ink adhesion. This approach, designed for manufacturing, provides valuable insights and offers field-proven solutions that prevent adhesion failures.
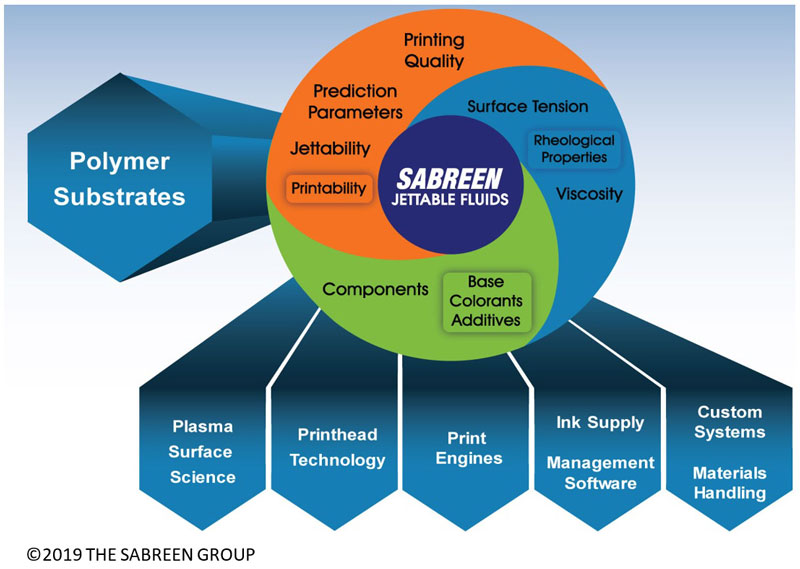
Inkjet printing is far more complex and delicate than analog printing. Ink-jetting of polymers is intrinsically difficult, since there often are substantial chemical and physical differences between plastics – even within the same polymer family. Printing on contoured geometries by inkjet adds further complications, unlike pad and screen printing solventborne ink formulations with naturally lower surface tensions that wet readily to polymer substrates.
Thermoplastics
The type of thermoplastic selected to be printed significantly affects the entire inkjet process. In a thermoplastic material, the very long, chain-like molecules are held together by relatively weak Vander Waals forces. Thermoplastics are divided into two subcategories, amorphous and crystalline (see Figure 2). Generally, amorphous polymers possess better adhesion properties than crystalline.
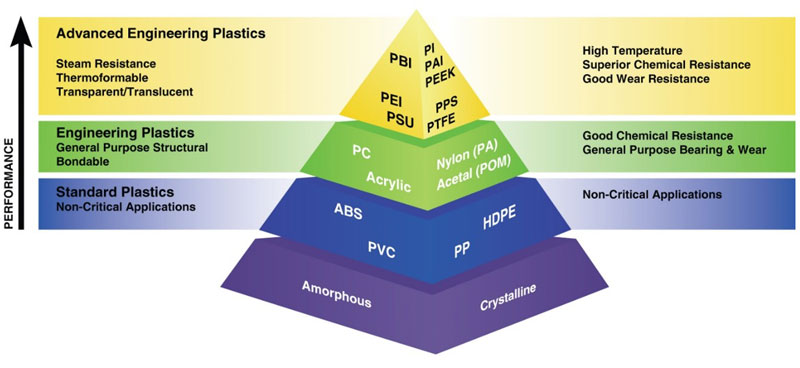
The term “amorphous” means to have no defined shape or an easily altered shape. “Crystalline” implies that there is a regular, defined pattern to the molecular aggregates. Amorphous resins exhibit random, spaghetti-like structures. They do not greatly dampen energy introduced into the materials. As heat is Inkjet Printing and Adhesion of Low Surface Energy Polymers applied, they soften and do not have a sharply defined melting temperature.
Crystalline resins have orderly patterns (see Figure 3). They also have well-defined melting temperatures. In a polymer, these two states coexist with adjacent section polymers packing into tight crystalline bundles held together by secondary attraction forces, while other sections of the same molecules are unable to physically move into the crystalline lattice and remain amorphous. The probability of a particular polymer segment being crystalline or amorphous is mostly a random event, controlled by the dynamics of the crystallization process.
Semicrystalline materials have a highly ordered molecular structure with sharp melt points. They do not gradually soften with a temperature increase; instead, semicrystalline materials remain solid until a given quantity of heat is absorbed and then rapidly change into a low-viscosity liquid. Amorphous polymers have a randomly ordered molecular structure that does not have a sharp melt point. Instead, amorphous materials soften gradually as the temperature rises. These materials change viscosity when heated, but seldom are as easy flowing as semicrystalline materials. Amorphous polymers lose strength quickly above their glass transition temperature (Tg).
Ink-polymer substrate compatibility
Among the most challenging polymers to inkjet print include: acetals (POM), polyamides (nylon), polyolefins, polyester (PET), polyphenylene sulfide (PPS), polyetheretherketone (PEEK), polyetherimide (PEI), polyimide “Kapton” (PI), polytetrafluoroethylene (PTFE) and polyurethanes (PUR). Designers select these low surface energy (LSE) materials for their lightweight metal replacement, stiffness and strength, chemical resistance and physical properties at elevated service temperatures.

For inkjet printing to be successful, it must achieve long term adhesion to the polymer surface. Adhesion depends largely upon surface phenomena, i.e., the elevated temperature ink must easily jet from the printhead and appropriately interact with the substrate. The ink must be able to make intimate contact with the surface of the polymer substrate. This intimate contact is termed wetting or wetting out the surface and refers to the ink’s ability to spread over the surface. Most polymer surfaces are naturally hydrophobic and resist being wetted. Characteristically, these plastics are chemically inert, nonporous surfaces with low surface energy, which makes printing adhesion nearly impossible. Surface energy is the excess energy that exists at the surface (as opposed to the bulk) of a solid. This excess energy exists because molecules at the surface cannot interact with as many like neighbors as molecules in the bulk are able to do; therefore, they have excess interaction energy.1
Primary processing is critical in order to achieve the stated properties of the polymer material. For example, examine two LSE polymers that are difficult to inkjet – polyamide (PA) and polyphenylene sulfide (PPS):
Polyamide, commonly known as nylon, is a semicrystalline polymer. The two most common grades are Nylon 6 and Nylon 6/6. Nylon polymers are inherently difficult to bond because they are hydrophobic, chemically inert and possess poor surface wettability (i.e. low surface energy). Further, nylons are hygroscopic and will absorb moisture in excess of 3% of its mass of water from the atmosphere. Moisture, in and of itself, creates adhesion problems. Proper drying procedure of nylon resins is critical to processing and part performance. Most noteworthy, the hydrophobic behavior of nylon is a surface property, and the hydrophilic behavior is a bulk property. Since nylon is an organic polymer, it has a relatively low surface energy. This is a consequence of the surface chemistry and surface physics of polymers and other organics. However, the amide groups in the nylon chain attract water, and they give rise to the hydrophilic behavior of this material in regard to bulk absorption of water. Thus, in the bulk, nylon can behave as a hydrophilic material, but on the surface, it can exhibit hydrophobic behavior.
Polyphenylene sulfide (PPS) offers the broadest resistance to corrosives of any advanced engineering plastic. PPS products are not hygroscopic and, therefore, do not experience dimensional expansion problems like nylon (polyamides). Yet it is important to use dry resin in molding parts. Moisture, in and of itself, is problematic. High moisture levels can create voids, which could adversely impact part performance, affect adhesion and alter aesthetics. The time between drying and processing should be as short as possible. PPS should be dried in dehumidifying hopper dryers. Hot air ovens are generally not recommended. To achieve a fully crystalline state, mold temperatures of at least 275 to 300°F are required. When PPS is molded below 275°F, the moldings are amorphous, or semicrystalline, and remain in this state until they are exposed to higher service temperatures (including heat curing). If the service temperature exceeds the molding temperature, the parts will become more crystalline, resulting in dimensional and property changes. For example, the heat deflection temperature (HDT), @264 psi (1.8 MPa), of 40% glass-filled PPS molded in a noncrystalline state is only 350°F but increases to >500°F (260°C) in the crystalline state. Further, mold temperature has a dramatic effect on the surface appearance.
To suitably clean nylon and PPS surfaces and remove LMWM (low molecular weight materials), use toluene, xylene, acetone or MEK. Alcohol is a weak solvent and only removes superficial dirt but not hydrocarbon contaminates. Proper technique must be used at all times, including using lint-free cloths and wearing powder-free protective gloves. Excess solvent creates weak boundary layers of unremoved chemicals, leaving a haze buildup that will inhibit bonding.2 Printing processes should be performed as soon as possible following molding operations, or package parts tightly in non-poly bags such as polyester. Nylon and PPS bonding applications often require plasma pretreatment, such as electrical corona discharge, electrical atmospheric plasma, electrical air plasma, flame plasma and low-pressure RF cold gas.
Clean substrate surfaces
Low molecular weight materials – such as silicones, mold release, anti-slip agents and process additives – inhibit the ink’s ability to flow and achieve intimate contact essential for adhesion. Certain soluble or nonsoluble compound agents used in pigment and dye colorants can adversely affect adhesion. Chemical makeup of the molded surface, texture and porosity significantly affect ink flow and adhesion. The degree or quality of treatment and adhesion are affected by the cleanliness of the plastic surface. The surface must be clean to achieve optimal pretreatment and subsequent ink adhesion. Surface contamination – such as silicone mold release, dirt, dust, grease, oils and fingerprints – inhibit treatment. Material purity is also an important factor. The shelf life of treated plastics depends on the type of resin, formulation and the ambient environment of the storage area. Shelf life of treated products is limited by the presence of low molecular weight oxidized materials (LMWOM), such as antioxidants, plasticizers, slip and antistatic agents, colorants and pigments, and stabilizers, etc. Exposure of treated surfaces to elevated temperatures increases molecular chain mobility – the higher the chain mobility, the faster the aging of the treatment. Polymer chain mobility in treated materials causes the bonding sites created by the treatment to move away from the surface. These components may eventually migrate to the polymer surface.
Mold tool design and surface texture
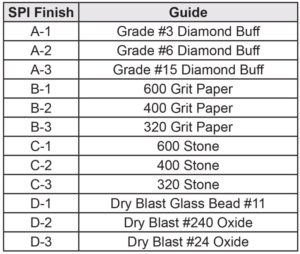
Designers can improve adhesion by adding texture to the mold surface(s). Texture introduces peaks and valleys, providing mechanical interlocking adhesion. Texture can be accomplished within the mold tool or manually using a Scotch-Brite pad and solvent cleaning. Molded-in texture is preferred because it maintains surface characteristics at no additional labor/chemical costs. For example, NTMA mold cavity Finish “40-Diamond buffed 1200 Grit” likely will improve bond strength vs. Finish “10-Fine Diamond 8000 Grit” (0 to 3 micron range). Reference Figure 4 SPI Mold Surface Finish. Even slightly textured surfaces are beneficial. For recessed-hole applications, like connectors, etched core pins in the mold are highly effective.
In summary, to achieve robust ink adhesion and/or high-strength adhesion bonding of polymers, the following items are recommended. None of these items add cost and, in fact, off er cost-savings.
- No internal or external silicones. Minimize processing additives.
- Mold texture.
- Properly dry the resin before molding.
- Surfaces must be clean.
- Avoid using excess solvent that creates weak boundary layers of unremoved chemicals.
- Package molded parts tightly in non-poly bags with a desiccant or use polyester bags.
- Conduct the printing process as soon as possible following molding.
References
- Innovations in Bonding to Low Surface Energy Surfaces, 3M Technical Guide
- Best Practices for Bonding Semi-Crystalline Thermoplastics, The Sabreen Group, January 2015
Scott R. Sabreen is founder and president of The Sabreen Group, Inc., an engineering company specializing in secondary plastics manufacturing processes – product security, laser marking, surface pretreatments, bonding, decorating and finishing. Sabreen has been pioneering technologies and solving manufacturing problems for over 30 years. He can be contacted at 972.820.6777 or by visiting www.Sabreen.com.



