by Arthur Kasson, technical consultant, and Frederick Fiddler, technical manager, KRUSS USA
Today’s plastic materials are tough, durable and lightweight, making them highly desirable in a wide range of manufacturing processes – aerospace, medical devices, automotive, electronics, semiconductors and more. Quality control and R&D professionals throughout these industries understand that when it comes to adhesion of plastic materials, there are some important challenges to consider, including surface treatment and cleaning processes. For instance, when ink-jetting onto a plastic surface, cleaning may be done by wiping the surface with alcohol. Or a plastic roll might be treated with in-line plasma or super-cooled CO2 liquid. It is worth taking a deeper look at how treatment methods and cleanliness of surfaces affects optimal adhesion.
Sample preparation and surface adhesion
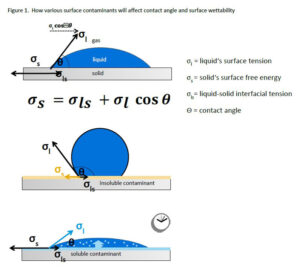
In order to understand the effect of treatment or cleanliness on a surface, it’s important to evaluate the interfacial chemistry, or the physical and chemical conditions that exist when two phases interact. Seeing this overall picture of the wetting process will allow for predicting work of adhesion and long-term bonding while also inhibiting delamination and adhesion failures.
To measure these parameters, look to Young’s equation, which defines the relationship between the contact angle of a liquid, the liquid’s surface tension, the liquid/solid interface and the solid’s surface free energy. When a contaminant, whether soluble (i.e., salt on metal) or insoluble (i.e., oil on plastic), is present, this can have a significant impact on the result (Figure 1).
What is surface tension and how is it measured?
Surface tension (SFT) of liquids is the result of imbalance forces due to greater attraction of the molecules at the liquid-air interface than the attraction of these same molecules to molecules in the air.
There are several methods used to measure SFT, and the SFT range often dictates which method is utilized, most commonly DuNouy Ring and Wilhelmy Plate and Pendant Drop methods.
Determining surface free energy
The procedure for determining surface free energy (SFE) involves placing a drop of water (a polar liquid) and diiodomethane (a disperse liquid) on the surface of the substrate, measuring the resulting contact angles of these drops, then using the contact angles to calculate the SFE of the substrate. The contact angles then can be used in different mathematical models.
Calculating work of adhesion and interfacial tension
Once the SFT, SFE (polar and disperse) measurements are made, the work of adhesion and interfacial tension (IFT) can be calculated. These parameters will affect the initial adhesion and longevity of the adhesion of coating liquids onto a given surface.
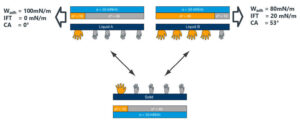
interactions between two liquids (A, B) and
one substrate. The resulting contact angles,
interfacial tension and work of adhesion
also are shown.
Figure 2 shows two liquids with the same SFT but with opposite polar and disperse component ratios. The polar and disperse components of both liquids and substrate are designated as the yellow and gray hands, respectively. When the liquid and solid come in contact, the liquid and the substrate with the highest interaction (liquid A) shows the highest work of adhesion and the lowest interfacial tension.
This suggests that liquid A and substrate combination will have a better initial adhesion and, over time, will be more stable than liquid B, which has minimal interaction with the substrate, lower work of adhesion and higher interfacial tension.
Coated fishing line: A case study
One of the challenges with fishing lines is delamination of the coating after multiple castings. In order to evaluate the cause, a characterization of high-strength Kevlar fishing lines and coating was performed.
Step 1: Determine SFT and SFE (polar and disperse components) of the fishing line and coating, using Pendant Drop method and water and diiodomethane contact angle, respectively. Calculate work of adhesion and IFT of the fishing line and coating.
Results: (Table 1) The SFT of the coating was lower when compared to the SFE of the fishing line, suggesting that the coating will wet the surface of the fishing line.
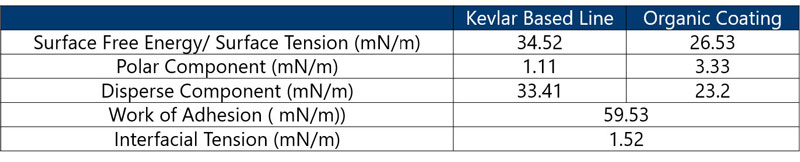
However, it showed that the polar component of the SFE of the fishing line was very low and the ratio of disperse and polar components was quite different from component ratios of the coating liquid.
Step 2: Treat the surface using plasma.
Results: (Table 2) As expected, the polar component of the SFE of the fishing line and the work of adhesion increased. A decrease in the interfacial tension was also observed.
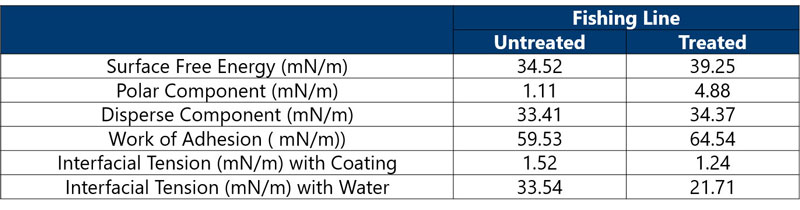
At first glance, it seems that plasma treating the fishing line surface would enhance the adhesion of the coating and longevity of the coating on the surface.
However, when the IFT between water and fishing line (before and after treatment) was calculated, it showed a decrease from 33.54 to 21.71 mN/m. After plasma treatment, the fishing line had a greater affinity for water and exacerbated the delamination of the coating by allowing water to creep between the cracks in the coating and the surface of the fishing line.
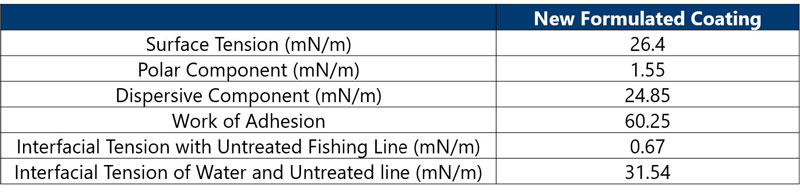
Step 3: Optimize the coating liquid to achieve the desired adhesion.
Result: (Table 3) The work of adhesion calculation showed that the reformulated coating had a high work of adhesion, low interfacial tension, better resistance to water and – after more than 250 castings – a decrease in coating delamination.

Arthur Kasson is a technical consultant for KRÜSS Scientific Instruments and covers sales responsibilities in the northeast US. He spent several years managing mass spectrometry labs at various universities before getting into sales.

Frederick Fiddler currently serves as the technical manager at KRÜSS USA.
KRÜSS is a specialist in interfacial chemistry and supplier of measuring instruments for surface and interfacial tension. The company offers customers first-class measuring instruments combined with practical, scientific knowledge transfer. For more information, visit www.kruss-scientific.com.
References
- KRŰSS Application Report 232, Work of Adhesion and Interfacial Tension.
- KRŰSS Application Report 286, Determine How Clean Surfaces Are: Quickly and On the Go.


