by Rose Roberts, Ph.D., senior custom applications and materials engineer, Brighton Science
Surface energy is the ability of molecules on a surface to attract other molecules. This is important for predicting when paints, coatings and other decorations will stick to a surface. Rose Roberts, Ph.D., with Brighton Science, provided insight into this important topic.
Explain the different methods available for measuring surface energy.
To be technically specific, there is no method that directly measures the surface energy of a solid material. However, the surface energy of a solid can be estimated through several means.
Perhaps the most precise method is a multiple probe fluid methodology called the Owens, Wendt, Rabel and Kaelble method or OWRK method (Figure 1). The method measures the contact angle of at least two fluids, preferably four or five, on a surface. Using these contact angles and each fluid’s polar and dispersive components of the surface tension, the polar and dispersive components of the substrate’s surface energy can be calculated. The total energy is the sum of these two components.
The choice of fluids used is important, as a wide range of polar and dispersive fluids should be chosen. The dispersive component measures van der Waals forces, which are present between all molecules. The polar component measures bonds such as hydrogen and ionic bonds where the electrons tend to be highly reactive. Not all molecules contain polar functional groups, but these tend to be the most reactive components of a molecule, if present. The most common fluid choices include water, diiodomethane, ethylene glycol, dimethyl sulfoxide and glycerol, as together they easily span the surface energies commonly found on most materials.
One relatively quick and commonly used method of estimating surface energy is called wetting tension, measured using a series of dyne solutions. These dyne solutions have a range of surface tensions, usually from about 30 to 72 dynes. A solution is spread across a surface via a swab or pen, and the liquid is observed to see if it remains spread across a surface (wetting) or if the liquid beads up in two seconds or less, as defined by ASTM D2578. If the liquid film stays continuous, the surface tension of the liquid is less than the wetting tension of the solid surface. An operator continues to try solutions with greater and greater surface tensions until the solution is found that just barely begins to bead up at the two-second mark. The surface tension of this solution defines the wetting tension of the surface. Note that this “wetting tension” parameter is not the same as total surface energy, although the two frequently can be correlated with each other.
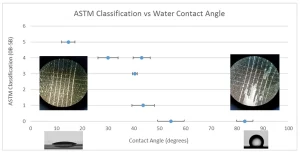
As the reader may have guessed, using dyne solutions is subjective: Two seconds is a very quick period of time to get a measurement, and it can be difficult to define “barely begins to bead up.” An alternative measurement is to simply use water contact angle.
Water contact angle is determined by both polar and dispersive components of surface energy, but it is especially sensitive to the polar component. As mentioned previously, the polar component generally is the most important factor to track in regard to reactive chemical functional groups for bonding purposes. When using a treatment or cleaning process, this polar component is the factor that changes the most and can be correlated with total surface energy. At Brighton Science, it has been found that nearly all customers’ painting, coating, bonding and other adhesion applications show a correlation between performance (e.g., adhesion) and water contact angle. Because of that, a tool was developed that will quickly and easily measure water contact angle on surfaces so industries can monitor and control their adhesion processes (Figure 2).
What are some potential complications of using wetting tension via dyne solutions to measure surface energy?
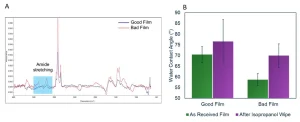
There are several complications that can occur when using dyne solutions (Figures 3 and 4). One of the biggest dangers for process control is that because these are solvent-based solutions, contaminants such as mold releases and blooming agents will dissolve into the dyne solution and give the operator a false reading. Only in cases of very large amounts of contamination on the surface will a dyne solution discover this issue, but many printing, painting and adhesive bonding processes only can tolerate small amounts of contamination.
Other complications include subjectivity of the test, as mentioned previously. Subjectivity further is exacerbated when the color or texture of the substrate makes it difficult to see the ink. Also, not all operators follow the ASTM standard, which makes it more difficult to get similar results between operators at the same facility and between facilities.
As described in the ASTM standard, the amount of solution applied to the surface during a wetting tension measurement is important. The felt of dyne pen markers can break down over time and deposit too much ink, which provides a false reading. Dyne pen markers also are extremely prone to becoming contaminated, which will give a false reading. Use the disposable swab method can reduce the possibility of contaminating the dyne solution if care is taken to not “double-dip” a swab in the ink.
Additionally, some dyne solution formulations use volatile components which can evaporate quickly and change the surface tension of the solution, especially if the bottle is left open for a bit or if the dyne pen is left uncapped for a short time.
Outside of process control dangers, there is a danger to the operators using dyne solutions. Most dyne solutions contain formamide, which may cause cancer, damage the reproductive systems or present a danger to an unborn child, as well as damage organs over time. These dangers can be mitigated if an alcohol-based dyne solution is used, but consult the dyne solutions’ Safety Data Sheets to check the content of the inks.
Explain estimation of surface energy via water contact angle and how to measure surface energy via water contact angle.
As mentioned above, water contact angle correlates very well with total surface energy. By using only water, the level of reactive chemical functional groups on a surface can be tracked, which is one of the most important factors for adhesion. The technique has been in use for a long time and, in fact, has been codified by the ASTM as an alternative to dyne solutions.
To measure water contact angle, the traditional method is to use a goniometer. Modern goniometers are desktop or handheld units attached to a computer and require a small, flat substrate that will fit on the sample stage. It measures the contact angle of the droplet from two opposing sides of the drop. This equipment can be unwieldy to use on many production lines, which may include complex geometries or moving lines. Also, for parts whose surface or shape may cause some ellipticity or other non-round shapes, it can be difficult to get an accurate reading representative of the truer average contact angle of the drop.
A newer method is embodied in a handheld, portable instrument called the Surface Analyst™, which deposits a droplet, measures the average diameter of that droplet from a top-down image, then calculates the average contact angle from the average diameter and the volume. This method gives an objective value for surface treatment level or cleanliness in approximately two seconds, is easy to use and the top-down measurement can fit most complex geometries. Custom-made inspection attachments can be created to fit into these geometries to ensure an accurate measurement. Besides the portable version, inline versions are available, as well as semi-automated quality inspection station versions. Pass/fail thresholds can be programmed into the tool so operators immediately know whether the surface is ready for bonding. If hydrophilic contamination is suspected on the surface, there is an algorithm to measure if dynamic wetting is occurring. Dynamic wetting indicates that a hydrophilic contaminant, such as a surfactant or rust preventative, is dissolving into the water, which changes the surface tension of the water itself and causes the droplet to spread out over the course of a few seconds.
What are the challenges with establishing strong, durable interface to thermoplastics?
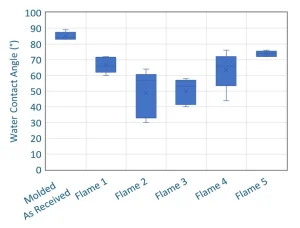
One of the major challenges to forming strong, durable bonds to thermoplastics is that many have a low surface energy with few chemically reactive groups. Adhesive bonds mostly are chemical in nature, and achieving a strong chemical bond requires active chemical functionality on both sides of the joint. Because of this, many thermoplastics require a surface activation process, such as flame, corona or plasma treatment. These processes are similar in that oxygen-containing functional groups are added to the surface of a material, raising the surface energy and increasing the likelihood to form a strong chemical bond.
How much treatment does a surface need? Every system will be different. Tracking the water contact angle and correlating with bond performance is the best method currently known to determine treatment level needed. This article specifies the water contact angle because this specifically tracks the oxygen-containing functional groups that are being added through the treatment process, as described previously. An easy place to start is to maximize treatment. Treat the surface until as low of a contact angle as possible is reached. If overtreated, some polymers will begin to show an increase in contact angle again because enough energy has been added to allow unbranched or non-crosslinked polymers to reorient and present an untreated portion of the molecule. For polymers that cannot reorient, the contact angle will simply plateau, and the point of just reaching the contact angle plateau is the maximum treatment.
When treating polymer surfaces, one important factor to keep in mind is surface cleanliness before treatment. Mold release, rust preventative on tooling and blooming agents all can interfere with treatment processes. It is imperative to check surfaces both before and after treating to ensure the surfaces are the same going into a treatment process so reliable results come out of the treatment process. Checking the parts after treatment ensures that the treatment equipment has not changed in any way over time. Dirty burners and equipment that is out of optimal alignment can contribute to unexpected treatment quality changes.
In addition to Surface Analyst™, Brighton Science can help perform root cause analyses on problem production lines, develop a bonding process (including setting up a treatment step) and other work related to improving the adhesion process. Dr. Roberts began working at Brighton Science in 2019 and now manages the consulting lab. Her specialties are helping customers define new adhesion processes and determine root cause current adhesion processes when they are failing. For more information, visit www.brighton-science.com.
References
- D.H. Kaelble, Dispersion-Polar Surface Tension Properties of Organic Solids, J. Adhesion 2 66-81 (1970).
- Boerio, Roby, Bossi, Dillingham, Crane, J. Adh. 82 19-37 (2006).
- R.J. Caimi, L.K. Derr, T.J. Dunn, Precision of the surface energy test, Converting Magazine, 10 (6) (1992) 62.
- M. Jin, F. Thomsen, T. Skrivanek and T. Willers, Why Test Inks Cannot Tell the Whole Truth About Surface Free Energy of Solids, Advances in Contact Angle, Wettability and Adhesion Volume 2, K.L. Mittal (ed.), Scrivener Publishing, 2015, pp. 419-438.
- ASTM D5946, Standard Test Method for Corona-Treated Polymer Films Using Water Contact Angle Measurements.
- M. Blitshteyn, Wetting tension measurements on corona-treated polypropylene films, TAPPI Journal, 78 (3) (1995) 138-143.


