by Scott R. Sabreen, president, The Sabreen Group, Inc.
Note from the author: Printing and adhesion problems on many polymers and rubbers are common throughout industry. This is a result of the inherently low surface energy of polymers, among other factors. Surface oxidation processes can oxidize polymer surfaces, making them more wettable.
Ultraviolet (UV)/ozone pretreatment is a highly effective photochemical oxidation process for removing organic contaminates from polymer surfaces and improving adhesion. The process is conducted at ambient conditions, easy to use and inexpensive. This article discusses UV/ozone pretreatment, its chemical mechanisms and application advantages.
Thermoplastics are non-polar, inherently hydrophobic, low surface energy substrates that generally do not adhere well to other like or dissimilar materials. This is essentially due to their weak boundary layers caused by impurities arising during the polymerization, low molecular weight species at the surface, additives such as antioxidants and slip agents, external processing aids like mold release agents and post-fabrication contaminants.
The most common surface modification of polymers is oxidation. While factors such as surface texture topography improve wetting, the use of plasma surface modification pretreatments to promote adhesion between difficult-to-bond plastic substrates solves many bonding problems. Plasma pretreatments include electrical corona, atmospheric plasma, remote-cold gas, flame and UV/ozone. These processes are characterized by their ability to generate “gas plasma,” an extremely reactive gas consisting of free electrons, positive ions and other species. Chemical surface functionalization also occurs. UV/ozone is generally regarded as milder than cold gas oxygen plasma due to the absence of high kinetic energy particles.
The effects of exposure to ultraviolet light and ozone with respect to polymer surface modification and adhesion have been practiced for decades. Scientific studies and industrial applications use combinations of UV light and ozone on specific polymers to maximize the treatment, i.e., UV light in air (producing ozone); UV light in air (producing ozone) supplemented by additional ozone in the incoming air; and ozone only.
The effectiveness of treatment is dependent on the properties of the exposed polymer and treatment processing. By examining the oxygen uptake levels, it can be determined how quickly that surface modification occurs.
UV/ozone pretreatment applications
The advantages of modern UV/ozone systems make them a preferred pretreatment for many 3D applications requiring wettability and strong adhesion bonding. The process is dry, conducted at ambient conditions, easy to use and inexpensive.
UV/ozone is ideal for maximizing capillary fluid flow in medical microfluidic devices and activating and functionalizing thermoplastic and rubber surfaces used in footwear products. Polymer applications benefitting from UV/ozone include ABS, COC, COP, EPDM, PDMS, PET, PMMA, polyolefins, SBS, polycarbonate, PVC and styrene.
UV/ozone treatment
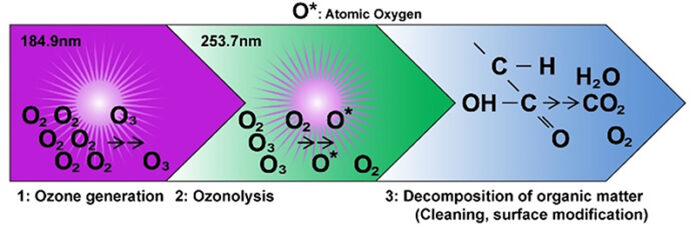
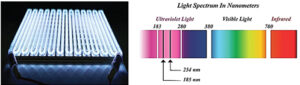
UV radiation is a small part of the electromagnetic spectrum, spanning from 100 to 400 nm. UV/ozone – or “UVO” – pretreatment is a photosensitized oxidation process in which molecules on either the substrate surface or organic contaminants are excited and/or dissociated by short-wavelength UV radiation absorption. UV radiation (wavelength = 185 nm) removes contaminants from surfaces by reacting with atmospheric oxygen to form atomic oxygen and ozone, both strong oxidizers. Ozone absorbs the 254 nm UV radiation and dissociates into molecular oxygen and atomic oxygen.
When polymeric molecules are exposed to UV radiation, they typically react with the atomic oxygen arising from the continuous dissociations of oxygen molecules and the generation of ozone molecules via the following mechanisms.
- Low-pressure mercury vapor discharge grid tube generates two wavelengths, 184.9 nm and 253.7 nm.
- The 184.9 nm UV decomposes oxygen molecules and synthesizes ozone O3. Changes of molecular oxygen to excited-state molecular oxygen O2* upon radiation at 184.9 nm.
- The 253.7 nm UV decomposes ozone and produces high-energy O* activated oxygen. Hydroxyl radicals also are produced in the presence of water vapor. Polymer substrates are oxidized by atomic oxygen, molecular oxygen and ozone through the abstraction of hydrogen atoms from the polymer chains, producing radical carbon sites. Hydroxyl and carboxyl groups likely are found on the polymer surface after the ozone treatment.
As ozone is being continually generated and destroyed, a steady-state concentration of atomic oxygen develops, where it acts as a strong oxidizing agent. The oxidation results in a more energetic surface, thus increasing hydrophilicity and enhancing wettability.
UVO pretreatment is greatly influenced by the effects of exposure time, UV intensity and distance from the UV source, ozone concentrations, gas flow rate and temperature. These effects depend greatly on the UV-absorbing characteristics of the polymer system, including fillers and additives. Not all polymers react the same way to UV irradiation.
Optimization of material parameters and processing conditions is always necessary to achieve the desired surface properties. Photooxidation in the presence of ozone has been shown in some studies on specific polymers and processing to be more effective than oxidation by ozone or UV light alone, whereas UV light alone is sufficient on other polymers.
Precleaning
UVO cleaning is generally effective for removing organic contaminates, though it may be ineffective for removing inorganic contaminates. It is recommended to preclean polymer substrates from dirt, dust and salts, which cannot be changed to volatile products by the oxidizing action.
Stability of UVO-treated polymers
The shelf life (treatment aging degradation) of treated plastics depends on the type of resin, formulation and the ambient environment of the storage area. Shelf life of treated products is limited by the presence of low molecular weight oxidized materials (LMWOM) such as antioxidants, plasticizers, slip and antistatic agents, colorants and pigments, stabilizers, etc. Exposure of treated surfaces to elevated temperatures increases molecular chain mobility. The higher the chain mobility is, the faster the aging. Pretreated surfaces age at different rates and to varying extents relative to factors with the surrounding environment. Activated surfaces may have a shelf life of hours, days, months or longer. Aging characteristics and their storage shelf life are essential to manufacturing process operations.
Some studies and application reports suggest that UVO-treated plastics may have shorter aging stability than other oxidative pretreatments, which may limit applications requiring long-term storage. New studies show UVO-treated plastics can last for months, and that storage condition has a significant impact on the surface stability. Specifically, the hydrophobic recovery of UVO-treated COC and PC can be inhibited by storing them in dehumidified or vacuum conditions, whereas the stability of PMMA is not significantly influenced by the storage condition. More importantly, these measurements show that the influence of the storage condition on the hydrophobic recovery is different for each type of plastic.
Conclusion
UV/ozone pretreatment is a highly effective photochemical oxidation process for removing organic contaminates from polymer surfaces and improving adhesion. The process is conducted at ambient conditions, easy to use and inexpensive. Optimization of material parameters and processing conditions is always necessary to achieve the desired surface properties. Wettability is greatly influenced by the effects of exposure time, UV intensity and distance from the UV source and ozone concentrations.
Not all polymers react the same way to UV irradiation. Studies show that photooxidation in the presence of ozone is more effective than oxidation by ozone or UV light alone, for some polymers. UVO-treated polymers and rubbers often have long shelf life. Shelf life of treated products is limited by the presence of low molecular weight oxidized materials and ambient storage conditions. Hydrophobic recovery of some plastics can be further inhibited by storing them in dehumidified or vacuum conditions. The influence of the storage condition on the hydrophobic recovery is different for each type of plastic.
Scott R. Sabreen is founder and president of The Sabreen Group, Inc., an engineering consulting company specializing in secondary plastics manufacturing processes – surface pretreatments, adhesion bonding, inkjet printing, laser marking, industrial coatings, decorating and finishing, and product security. He has been developing pioneering technologies and solving manufacturing problems for more than 30 years. He can be contacted at 972.820.6777 or by visiting www.sabreen.com.
References
- Keller RW. In: Cheremisinoff NP, editor. Handbook of polymer science and technology. Oxidation and ozonation of rubber, Vol. II. New York: Marcel Dekker; 1989.
- Tung-Yi Lin et al. Stability of UV/ozone-treated thermoplastics under different storage conditions for microfluidic analytical devices. The Royal Society of Chemistry, 2017
- Man Lung Sham et al., Cleaning and Functionalization of Polymer Surfaces and Nanoscale Carbon Fillers by UV/Ozone Treatment: A Review, 2009
- Mark Strobel, Mary Jane Walzak, Josephine M. Hill, Amy Lin, Elizabeth Karbashewski & Christopher S. Lyons (2012) A comparison of gas-phase methods of modifying polymer surfaces, Journal of Adhesion Science and Technology, 9:3, 365-383, DOI: 10.1163/156856195X00554
- Scarlett Wang, How to UV Ozone Cleaning, 2014
- Scott R. Sabreen, The Sabreen Group, Inc. “Plasma Surface Pretreatments of Polymers for Improved Adhesion Bonding,” Plastics Decorating magazine, 2018